Among the many things to keep top of mind as an Amazon seller, federal regulations on goods should be one of them. You could have an amazing product that checks off all the boxes for making a profit—low competition, affordable manufacturing costs, high demand—but if it’s not approved by the U.S. Food and Drug Administration (FDA), it can’t be sold.
That’s why understanding the implications of FDA compliance upfront is so important. It helps you avoid unwanted financial and legal repercussions, as well as ensure what you’re selling is safe. In this guide, we’ll cover everything you need to know about selling Amazon FDA-regulated products, from the importance of the rules and restrictions put in place to best practices for staying compliant. Let’s get started!
Why FDA Regulations Matter for Amazon Sellers
Can you sell non-FDA approved products on Amazon? The short answer is no, as the platform requires all products to have FDA approval before being listed. Much like other regulations, ones put forth by the FDA are designed to protect customers and your business. That being said, here are some key reasons why you should take them seriously.
- Legal Compliance: Selling products that aren’t FDA-compliant could result in legal fines, bans, or even criminal charges if the violation is severe. It’s all the more reason to abide by the rules.
- Account Suspension: In addition to the legal repercussions of selling non-FDA compliant products, Amazon can remove product listings or suspend your seller account for violating their policies.
- Consumer Trust: When it comes to purchasing products, customers rely on FDA regulations as a mark of safety and quality. Selling products that are FDA certified gives customers the assurance that they’re making a safe and trustworthy purchase.
- Brand Reputation: Selling products that are FDA certified can likewise help build a brand’s following and reputation as customer trust grows over time. Think of it this way; a brand that constantly deals with product recalls from violating FDA policies is likely to have a less favorable reputation among customers compared to a brand that sells FDA certified products.
Am I Selling FDA Regulated Products?
So, what products need FDA approval? Generally speaking, the FDA regulates food, beverages, drugs, biological products (e.g. blood products, vaccines), medical supplies and devices, radiation-emitting electronic products, cosmetics, tobacco products, and animal drugs. Likewise, all of these products need FDA approval and fall into a number of the Amazon restricted categories.
Categories that require the most regulation from the FDA – tobacco products, drugs, biological products, animal drugs and foods – are outright prohibited or require special permission to sell on Amazon. These restricted products may also have additional requirements from Amazon other than those stated in this article. The e-commerce platform takes restricted categories very seriously, so if you created an Amazon product listing without the proper FDA approval or complying to listing guidelines for that category, you could permanently lose your selling privileges.
Which Products are FDA-Regulated on Amazon?
Now that you generally know which products are FDA regulated, it’s time to go over the Amazon FDA-regulated products; that is, which restricted products you can find and sell on Amazon. In this section, we’ll cover a number of the Amazon restricted categories and provide some examples for each one.
Restricted Product Categories & Examples
| Product Category | Examples | Reason for Restriction | Additional Information |
| Food & Beverages | Products containing horse meat, goat’s milk infant formula, betel nut products or products containing betel nut flavoring | To ensure that any food or beverage sold is safe, authentic, and legal (i.e. complies with FDA regulations). | Full Amazon guidelines |
| Drugs & Medications | 1,4-Butanediol, Ephedrine, Ketamine | A drug is a substance used to diagnose, cure, treat, or prevent diseases in people or animals. Drugs can also be used to change the structure or function of the body (e.g. treating acne). Meanwhile, “controlled substances” are drugs that are illegal (e.g. cocaine or heroin). Products that are used with controlled substances may be considered drug paraphernalia. Such products are either prohibited or restricted on Amazon to protect public health and ensure they follow FDA regulations. | Some drugs require a prescription for sale, such as most antibiotics. Other drugs can be sold without a prescription if they’re already approved by the FDA to be sold over the counter. Full Amazon guidelines |
| Medical Devices & Accessories | Diabetic test strips that are pre-owned or not authorized for sale in the U.S., products that contain mercury (e.g. thermometers and batteries), certain menstrual cups (e.g. MonzcareR-cup) | A medical device is an instrument, apparatus, machine or related object used to diagnose, cure, treat, or prevent diseases in people or animals. They can also be used to change the structure or function of the body (e.g. stimulating hair growth). The FDA regulates medical devices intended for human use to ensure they are safe and effective. | Some medical devices may be sold over-the-counter (OTC) to general consumers if they are appropriately described and labeled. All products that have not been cleared for OTC use, are considered dangerous, or have been recalled by the FDA are barred from being sold on Amazon. Some products ride a fine line between being considered a medical device or not. To avoid breaking FDA regulations, both the seller and manufacturer must be careful of how they make and describe the product. Full Amazon guidelines |
| Cosmetics | Corrective and cosmetic contact lenses, Latisse, products with trichloroacetic acid | Some cosmetics may count as drugs and therefore require FDA approval. Products that intend to make people more attractive are generally classified as a drug or even a medical device. | Amazon also prohibits the sale of cosmetics that have been determined to present “an unreasonable risk of injury or illness” to users. Full Amazon guidelines |
| Tobacco Products | Cigarettes, cigars, e-cigarettes (whether or not they contain nicotine) | Tobacco use is the leading preventable cause of disease and death in the United States. Since 2009 through the Tobacco Control Act, the FDA has gained the authority to regulate tobacco products to protect public health. | Full Amazon guidelines |
| Animal Products | Animal fur or feathers from federally endangered species (e.g. cheetah, tiger), vaccines (e.g. rabies, lyme disease), products containing ivory | Animal products are regulated by the federal and local laws to protect animal welfare, prevent illegal trade, and ensure customer safety. Amazon likewise restricts or prohibits the selling of certain animal products to comply with these laws and regulations. | The seller must comply with all applicable federal and local laws when selling animal products. The seller is also responsible for obtaining any required licenses and permits and is liable for any penalties resulting from non-compliance. Full Amazon guidelines |
| Dietary Supplements | Acai Berry Coffee, Botanical Slimming, ZR Dietary Capsules | Dietary supplements do not require FDA approval before going on the market. The only exceptions are supplements that contain a new dietary ingredient, in which case the manufacturers are required to notify the FDA at least 75 days before selling. However, Amazon restricts or prohibits a number of dietary supplements since they do not meet the compliance checklist. | Amazon requires that supplements be correctly described and labeled. They also cannot make structure-function claims on their labeling or listing, unless the claim has been approved by the FDA. Structure-function phrases make claims that the product can affect the structure of the body (e.g. “reduces pain” or “anti-bacterial”). If the label includes such claims, the manufacturer must submit a notification to the FDA and include a disclaimer on the label that states the product has not been reviewed by the FDA and is not intended to diagnose, cure, treat, or prevent any disease. Any content on the listing that includes such claims must also have the disclaimer. Full Amazon guidelines |
For more information, you can view the full list of Amazon restricted categories here.
FDA Compliance Requirements for Products
In addition to knowing which products you can and cannot sell on Amazon, you should also understand FDA packaging and product label requirements.
Labeling Requirements
All Amazon products, even those that don’t require FDA approval before being sold, must follow certain labeling guidelines:
- Labels must not state that products cure, mitigate, treat, or prevent a disease (including conditions such as dandruff and acne) in humans unless that statement is approved by the FDA
- Labels must not state that products are “FDA Approved” if they are not FDA approved
- Labels must not use the FDA logo
One of the biggest mistakes a seller can make is to use the FDA logo on their product packaging to make it appear FDA certified. However, the FDA logo is for federal government use only. Displaying it on a product, product label, or listing detail page may violate federal law and will likely lead to the removal of the listing from Amazon as well as to possible legal consequences. Below is an example of a product listing that was removed by Amazon for violating this guideline as it suggested that the FDA endorsed the product.
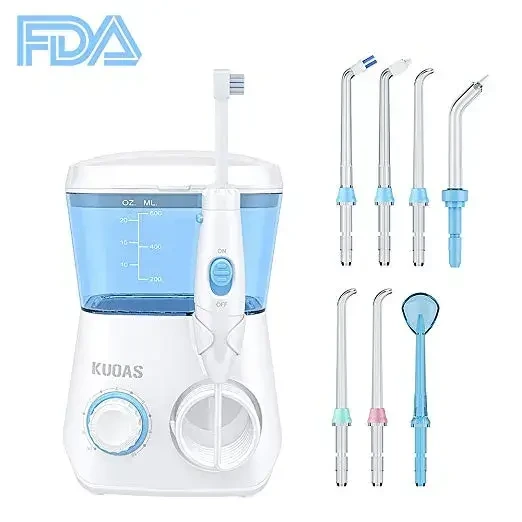
As an Amazon seller, you should ensure that you and your manufacturers understand these Amazon labeling requirements, especially if you don’t get a chance to see the finished product until it has already been manufactured. Although it can be tempting to use the “FDA Approved” symbol to advertise the safety and effectiveness of your product, misleading buyers can lead to negative reviews and returned orders, causing your seller account to be potentially suspended. Instead, you should leverage listing optimization and keyword optimization strategies to promote your product’s functionality and boost sales.
Packaging Requirements
To comply with Amazon FDA regulations, packaging must include the following:
- Product name
- Manufacturer / Distributor information (including name and address)
- Net content quantity
- Expiration date
- Ingredient list
- Allergen information (if applicable)
- Nutrition facts label (for food products)
- Any necessary warning labels
Additionally, all packaging should be designed to indicate if the product has been tampered with. For example, including a tamper-evident seal would make it easy for a customer to identify if their package has been opened or tampered with before its delivery.
Lastly, remember that depending on the product category you’re selling in (e.g. food, cosmetics, dietary supplements), additional specific packaging requirements may apply. Be sure to check other Amazon and FDA guidelines to ensure that you’re complying in all necessary aspects.
Compliance Documentation
To comply with FDA regulations, Amazon sellers typically need to provide documents such as the following:
- Certificate of Analysis (CoA): This document proves that a product meets the specified quality standards and is tested by a recognized laboratory.
- Product Labels: Accurate labels with all required and applicable information including ingredients, nutritional facts, and any necessary warnings according to FDA regulations.
- Manufacturing Records: Documentation detailing the production process, such as quality control measures.
- FDA Registration Number: For certain product categories, sellers may need to provide their FDA registration number.
- GMP (Good Manufacturing Practices) Certification: If applicable to the product category, sellers may need proof of adherence to GMP standards.
If you aren’t sure if you need to provide compliance documents to Amazon, you can check the product requirements in the Amazon Compliance Reference tool on your Seller Central account, or you can do your own research. The Compliance Reference tool is used to determine whether or not a product is subjected to any compliance requirements, understand specific product requirements, and identify the documentation needed to submit to Amazon. To access the Compliance Reference tool, follow the steps below:
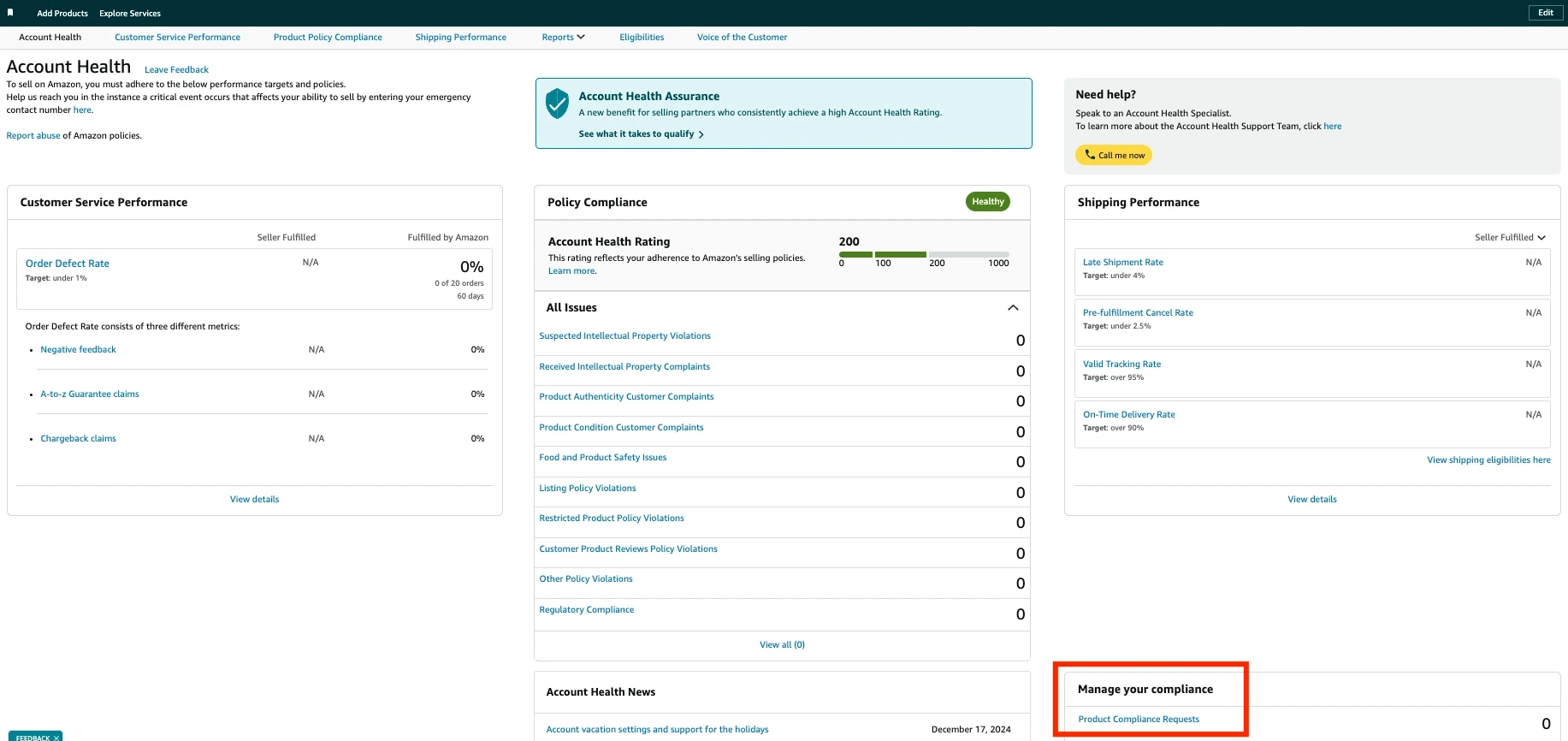
- Navigate to Performance > Account Health.
- Go to Manage Your Compliance and click Product Compliance Requests.
- In the upper menu, select the Compliance Reference tab.
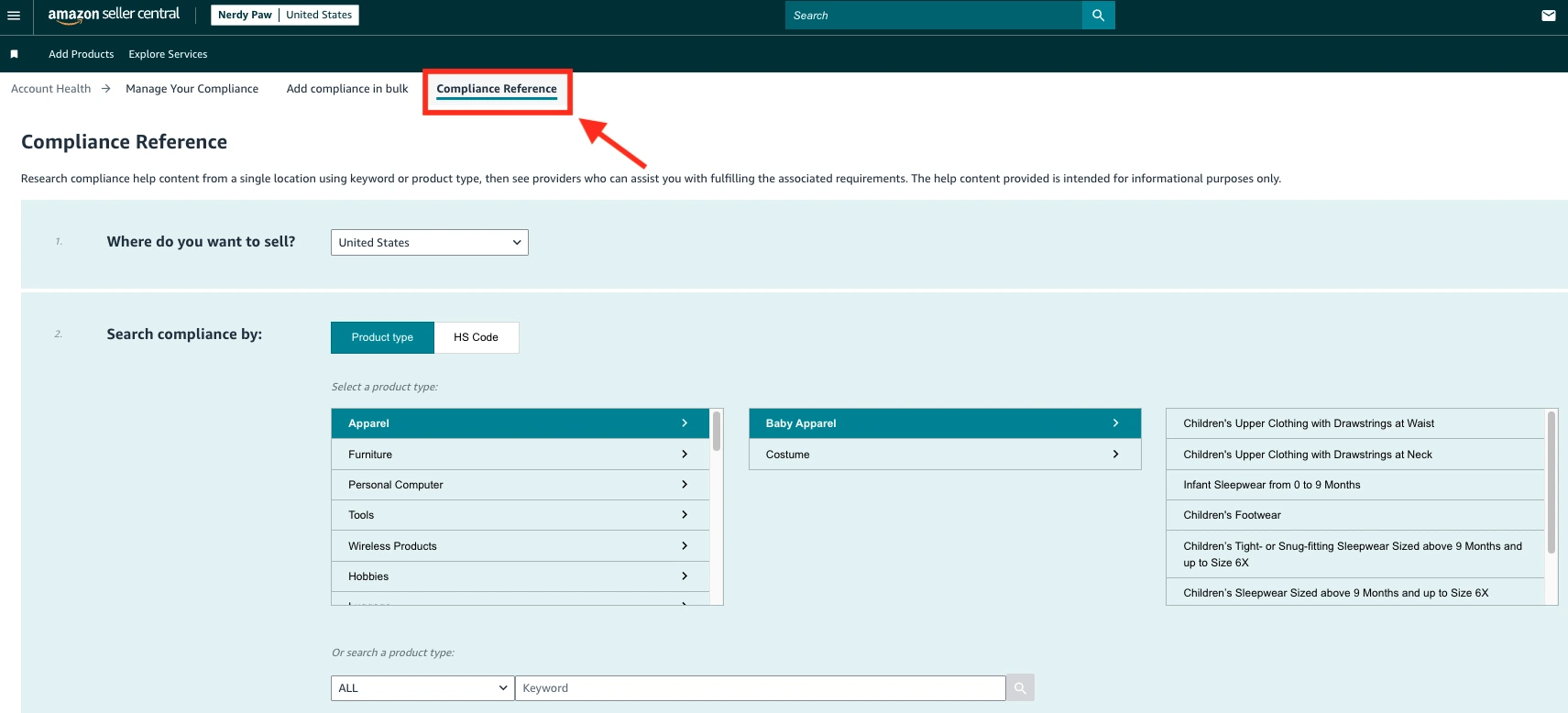
- Read and confirm the disclaimer, and navigate to the Compliance Reference tool.
- Enter the region you ship from and the store where you want to sell.
- Find your product category and keywords or enter the HS (Harmonized System) code. You will then see what compliance documents you need. Upload all necessary compliance documents.
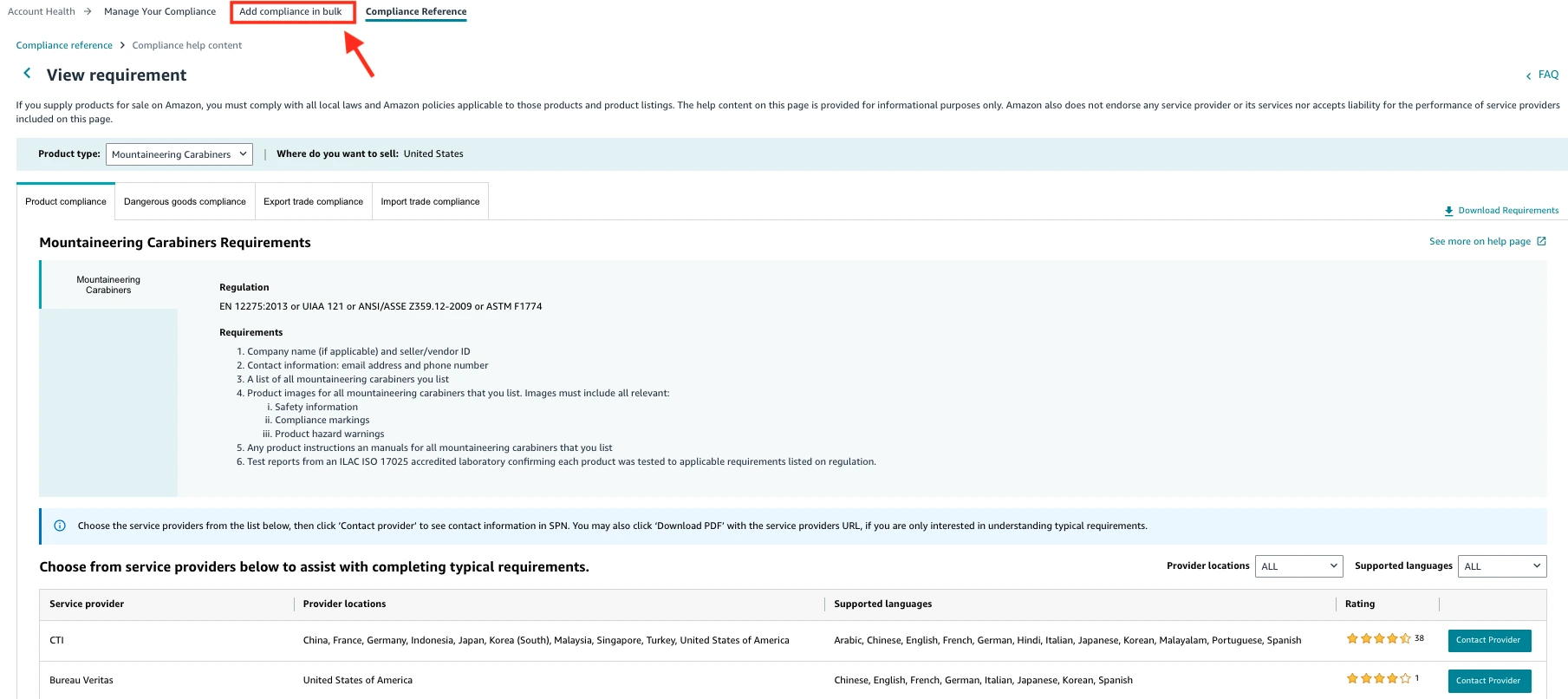
- If you don’t have any documents, the tool will show a list of providers that can help you get them.
Best Practices for Record Keeping
When maintaining all your documents, it’s best to keep things updated and organized. Here are a few tips to consider when handling your records:
- Use a digital document management system: Utilize a dedicated system to store and organize all compliance documents electronically. This makes it easier for you to readily access them for review and retrieval.
- Perform regular updates: Stay informed about any changes in FDA regulations and Amazon policies that might affect your product compliance. Make any necessary changes and update your documents accordingly.
- Conduct internal audits: Perform regular internal audits to identify potential compliance gaps and promptly address them.
Listing FDA-Regulated Products on Amazon
Selling Amazon FDA-regulated products can be a bit of a hassle with all the necessary checks and approvals you have to go through, but it’s not impossible. In this section, we’ll go over how to list FDA-regulated products on Amazon and relevant compliance policies.
How to List FDA-Regulated Products
- Check FDA compliance: Before you even begin creating a product listing, you should verify that your product meets all relevant FDA regulations for its category (e.g. food, cosmetics, drugs, medical devices) including labeling and packaging requirements.
- Log into or create an Amazon Seller Central account: Log into your Amazon Seller Central account or create one if you don’t already have one.
- Select a product category: Choose the correct product category on Amazon that matches your FDA regulated product.
- Provide accurate product details: In addition to providing product specifications and regular descriptions, you should also provide detailed product information such as ingredients, usage instructions, and warnings on your listings.
- Upload clear product images: Provide high-quality product images that comply with Amazon requirements.
- Add compliance statements: If applicable, include a statement indicating that your product is FDA compliant.
- Provide necessary documentation: If required, provide your FDA registration number on your listing. For products like cosmetics, you may need to provide safety testing reports to prove that your products were safely tested by a qualified laboratory. In addition, you may need to provide a manufacturer invoice in some cases to verify the source of your product.
- Wait for approval: Once you submit all necessary compliance documents, all you need to do is wait for Amazon’s approval before you can officially list your product.
Remember that some Amazon FDA-regulated products fall under restricted categories which require additional approval to sell. Always check Amazon seller policies to stay updated on specific requirements and ensure that your product listings meet all necessary Amazon and FDA guidelines.
To make things easier, you can also leverage a product listing analyzer to help evaluate the quality and performance of a listing. Viral Launch’s Listing Analyzer tool helps sellers monitor their listings in real-time and provides optimization, content evaluation, competitor analysis, and automated keyword discovery functions. Using a tool like this can help give you the assurance you need that your listings are optimized and compliant as needed.
Amazon Compliance Policies
As stated in the Seller Central Help section regarding the Restricted Products policy, all sellers “must comply with all applicable laws, regulations, standards, and [Amazon’s] policies related to those products and product listings. The sale of illegal products, or other products identified on [Amazon’s] Restricted Products policy page, is strictly prohibited.”
Ultimately, it is the seller’s responsibility to review the policies before creating or listing a product for sale. The seller is also responsible for ensuring that their products comply with all applicable federal and state laws, even if the product is not specifically described in Amazon’s policies. Since laws, regulatory requirements, and standards are subject to change, sellers should regularly monitor their listing for any legal or regulatory changes and make any necessary adjustments.
Any seller who violates Amazon’s policies or any applicable laws may be subject to an enforcement action, which can vary depending on the type of offense and the seller’s account history. Amazon considers a number of factors in the seller’s overall account when determining the course of action to take, including the severity and number of violations present on their account. Sellers who have had their selling privileges removed can appeal the decision in most cases. In some cases, Amazon may reinstate a seller’s account if they have addressed the root causes that led to their violations and implemented strong measures to prevent future violations.
If you have any current violations, you can view them in the Sellers Account Health Tab in Seller Central under AHD.
Best Practices for Compliance
Ensuring that your product passes Amazon and FDA compliance can be a hassle, but it’s important to establish good compliance habits to avoid potential violations. Below are a few best practices when it comes to Amazon and FDA compliance:
- Regularly review FDA updates: Stay informed about any changes in FDA regulations and make necessary changes in your product and product listings and packaging.
- Conduct quality control and tests: Implement quality control measures to set standards for manufacturing your product. You should also conduct product tests to double check for compliance and ensure that you meet all FDA guidelines and regulations.
- Work with trusted suppliers: Select reliable suppliers to ensure compliance. It’s also a good measure to get supplier verification and check a supplier’s credentials and compliance history before agreeing to work with them; this will save you from trouble later on if you run into potential violations.
Final Thoughts on Selling Amazon FDA-Regulated Products
Selling a product is no easy task and there’s a lot to keep track of, especially if you don’t manufacture your own product. However, simply staying updated on the latest changes in FDA guidelines can go a long way down the road and help avoid violations and potential legal trouble. Additionally, conducting proper product research can give you the assurance you need before you launch your online business in a specific product category and market. Whatever product you’re pursuing or decide to pursue, just make sure to stay informed on the latest news and trends!
Additional Resources
- For more information on how to get products FDA approved, visit https://www.fda.gov/
- For more information and resources about Amazon’s Restricted Products policy, visit the help section on Seller Central
- Learn how to get approved to sell FDA restricted products on Amazon by reading our article on Everything You Need to Know About Amazon Gated Categories
- For more information on e-commerce laws and regulations, check out our article on 10 E-commerce Laws and Regulations to Know Before Selling Online








